Learning Outcomes
By the end of this lesson, students will be able to:
i. Define and explain the concept of the periodic law, a fundamental principle in chemistry.
ii. Describe the periodic recurrence of chemical and physical properties of elements as a function of their atomic numbers.
iii. Recognize the contributions of Dmitri Mendeleev and his periodic table in establishing the periodic law.
iv. Apply the periodic law to predict and explain trends in the properties of elements, such as electronegativity, ionization energy, and atomic radii.
v. Appreciate the significance of the periodic law in organizing and classifying elements based on their similarities.
Introduction
The realm of chemistry is filled with diverse elements, each with unique properties and characteristics. Yet, amidst this diversity, there lies an underlying order, a pattern that governs their behavior. This pattern, known as the periodic law, provides a unifying framework for understanding the properties of elements.
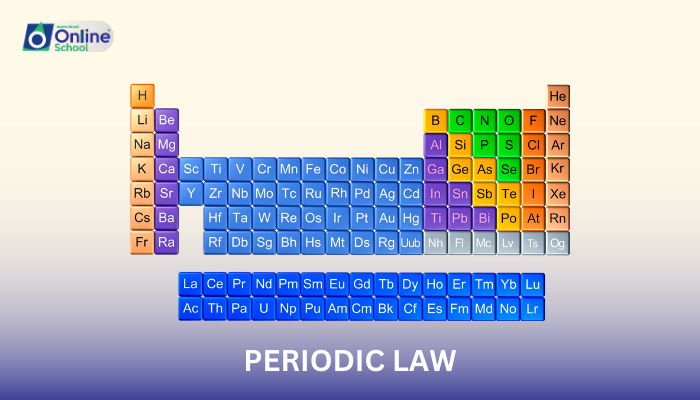
i. The Periodic Law: A Guiding Principle
The periodic law, a cornerstone of chemistry, states that the chemical and physical properties of elements exhibit a periodic recurrence when arranged in order of increasing atomic number. This means that elements with similar properties tend to appear at regular intervals within the periodic table.
ii. Dmitri Mendeleev and the Birth of the Periodic Table
Dmitri Mendeleev, a Russian chemist, played a pivotal role in establishing the periodic law. In 1869, he developed the first comprehensive periodic table, arranging elements based on their increasing atomic weights. Mendeleev's table, though not entirely accurate due to the then-limited understanding of atomic structure, demonstrated the periodic recurrence of element properties.
iii. Periodicity in Properties: Unveiling the Patterns
The periodic law manifests itself in various properties of elements:
Electronegativity: Electronegativity, the measure of an atom's ability to attract electrons, generally decreases down a group and increases across a period from left to right.
Ionization Energy: Ionization energy, the energy required to remove an electron from an atom, generally increases across a period from left to right and decreases down a group.
Atomic Radii: Atomic radii, the measure of an atom's size, generally decrease across a period from left to right and increase down a group.
These trends reflect the increasing nuclear charge and the filling of electron shells as atomic number increases.
iv. Significance of the Periodic Law
The periodic law holds immense significance in chemistry:
Organizing Elements: It provides a systematic arrangement of elements based on their similarities, aiding in classification and understanding.
Predicting Properties: It allows us to predict and explain trends in element properties, facilitating the study of chemical behavior.
Understanding Chemical Reactions: It sheds light on the basis of chemical reactions, enabling us to predict and explain the formation of compounds.
The periodic law, a cornerstone of chemistry, unveils the underlying order amidst the diversity of elements. By understanding the periodic recurrence of properties and the significance of atomic number, we gain a deeper appreciation for the organization and behavior of elements, paving the way for further exploration in the fascinating realm of chemistry.